OUR PROCESS
At the London Biofoundry, we develop the platforms and workflows to take any idea in biology through to the next stage.
As part of the Global Biofoundries Alliance, we are committed to the Design-Build-Test-Learn cycle.
We have developed our complete SynBio stack and can execute many builds and experiments in parallel fom DNA synthesis all the way to analytics in house.
Enter & exit
with purpose
Build
Results
Draw multiple conclusions
Design in
multiple streams
High throughput
experimentation
Big data
analytics
Learn and evaluate

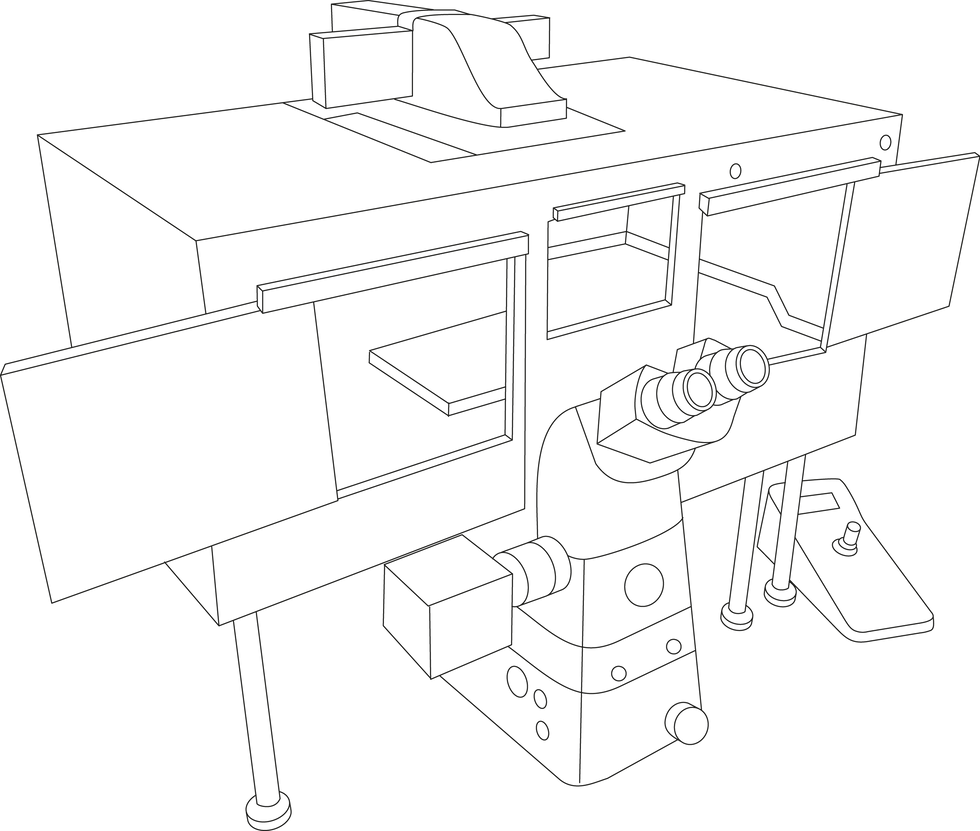
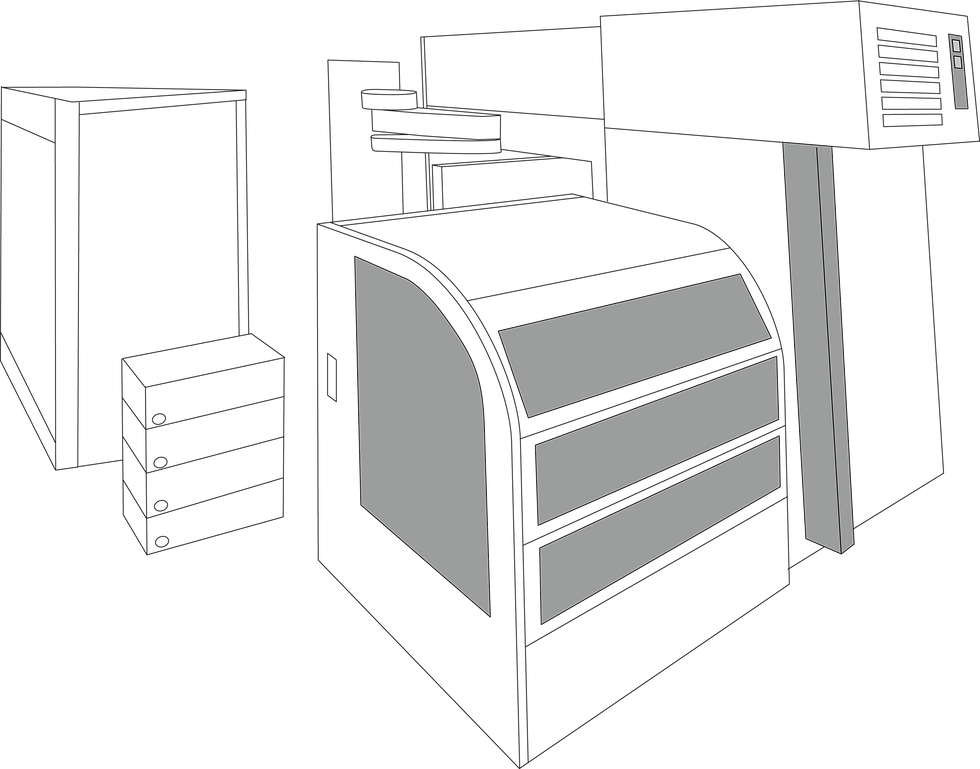





+
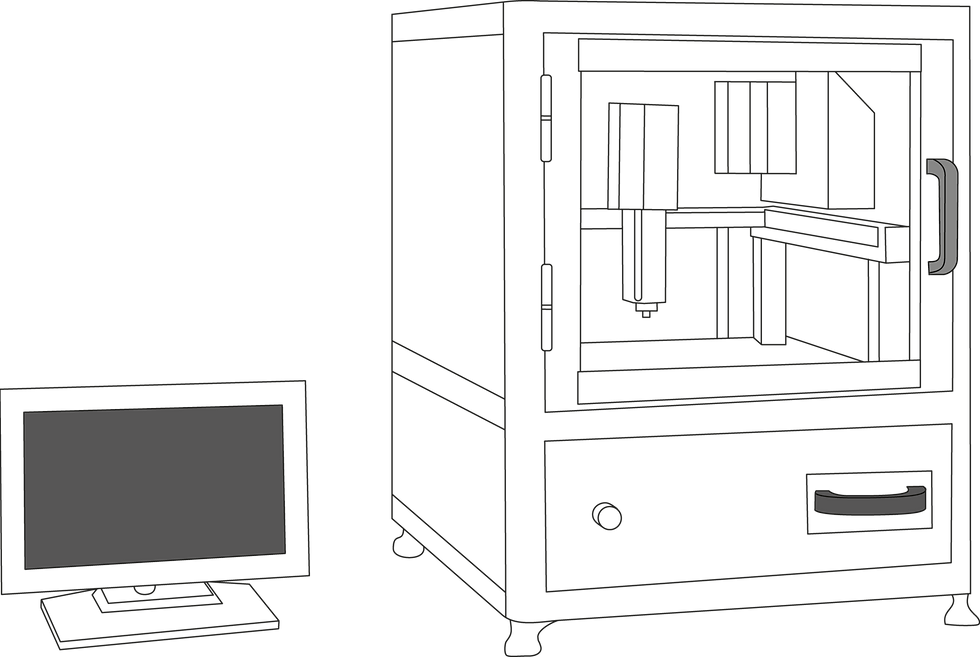



+
Customise your workflow
The London Biofoundry's response to the global pandemic

The SARS-CoV-2 pandemic has shown how the rapid rise in demand for patient and community sample testing, required for tracing and containing a highly infectious disease, has quickly overwhelmed testing capability globally. With most diagnostic infrastructure dependent on specialised instruments, their exclusive reagent supplies quickly become bottlenecks in times of peak demand, creating an urgent need for novel approaches to boost testing capacity. We address this challenge by refocusing the full synthetic biology stack available at the London Biofoundry onto the development of alternative patient sample testing pipelines. We present a reagent-agnostic automated SARS-CoV-2 testing platform that can be quickly deployed and scaled, and that accepts a diverse range of reagents. >Press release
Using an in-house-generated, open-source, MS2-virus-like-particle-SARS-CoV-2 standard, we validate RNA extraction and RT-qPCR workflows as well as two novel detection assays based on CRISPR-Cas and Loop-mediated isothermal Amplification (LAMP) approaches. In collaboration with an NHS diagnostic testing lab, we report the performance of the overall workflow and benchmark SARS-CoV-2 detection in patient samples via RT-qPCR, CRISPR-Cas, and LAMP against clinical test sets. The validated RNA extraction and RT-qPCR platform has been installed in NHS diagnostic labs with a testing capacity of 1000 samples per day and now contributes to increased patient sample processing in the UK while we continue to refine and develop novel high-throughput diagnostic methods. Finally, our workflows and protocols can be quickly implemented and adapted by members of the Global Biofoundry Alliance and the wider scientific and medical diagnostics community. >Article

In a study of infections in UK care homes with our colleagues at the Dementia Research Institute (DRI), we used our novel diagnostic testing platform to test residents for SARS-CoV-2 infection. We found a high rate of SARS-CoV-2 positive residents and our colleagues at the DRI realised that many of them did not show typical symptoms. Further, we extended our workflows by integrating the next generation sequencing workflows described by the ARTIC consortium to sequence the genomes of positive samples. Based on the sequencing data we showed that it was possible to identify clusters of infection based on differences in the genomes of SARS-CoV-2 infecting patients. >Article
With our partners we developed a novel sequening protocol for SARS-CoV-2 that replaces the typical complexity of sample preparation with a single-pot approach for sequencing library generation, making high-throughput viral sequencing much more practical and accessible. >Article

We previously showed how useful VLPs encapsulating the target RNA regions of the SARS-CoV-2 are for the development, validation and quality control of diagnostic workflows for Covid-19. We are working hard to make them available to researchers, who are also striving to develop and deploy novel diagnostic workflows to cope with the persisting shortage of testing capacity - essential to the the test-trace strategy recommended by the WHO.
.png)

